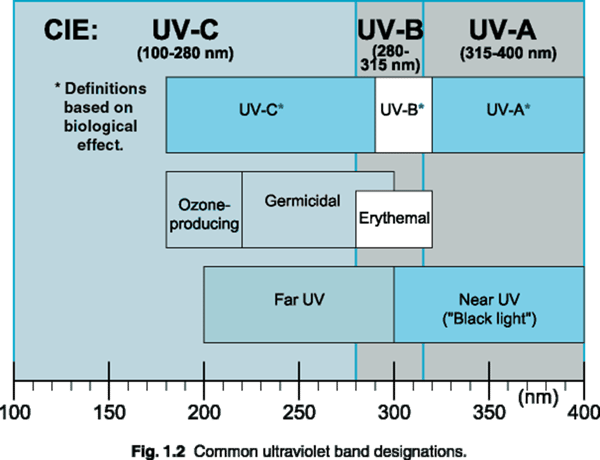
Short wavelength UV light exhibits more quantum properties than its visible and infrared counterparts. Ultraviolet light is arbitrarily broken down into three bands, according to its anecdotal effects.
UV-A is the least harmful and most commonly found type of UV light, because it has the least energy. UV-A light is often called black light, and is used for its relative harmlessness and its ability to cause fluorescent materials to emit visible light - thus appearing to glow in the dark. Most phototherapy and tanning booths use UV-A lamps.
UV-B is typically the most destructive form of UV light, because it has enough energy to damage biological tissues, yet not quite enough to be completely absorbed by the atmosphere. UV-B is known to cause skin cancer. Since most of the extraterrestrial UV-B light is blocked by the atmosphere, a small change in the ozone layer could dramatically increase the danger of skin cancer.
Short wavelength UV-C is almost completely absorbed in air within a few hundred meters. When UV-C photons collide with oxygen atoms, the energy exchange causes the formation of ozone. UV-C is almost never observed in nature, since it is absorbed so quickly. Germicidal UV-C lamps are often used to purify air and water, because of their ability to kill bacteria.
Question: What Materials Glow Under a Black or Ultraviolet Light?
From Anne Marie Helmenstine, Ph.D.,
ANSWER: There are a lot of everyday materials that fluoresce, or glow, when placed under a black light. A black light gives off highly energetic ultraviolet light. You can't see this part of the spectrum, which is how 'black lights' got their name. Fluorescent substances absorb the ultraviolet light and then re-emit it almost instantaneously. Some energy gets lost in the process, so the emitted light has a longer wavelength than the absorbed radiation, which makes this light visible and causes the material to appear to 'glow'.
Fluorescent molecules tend to have rigid structures and delocalized electrons. Examples of common materials that contain fluorescent molecules include:
- Club Soda or Tonic Water
The bitter flavoring of tonic water is due to the presence of quinine, which glows blue-white when placed under a black light. - Body Fluids
Many body fluids contain fluorescent molecules. Forensic scientists use ultraviolet lights at crime scenes to find blood, urine, or semen (all fluorescent). - Vitamins
Vitamin A and the B vitamins thiamine, niacin, and riboflavin are strongly fluorescent. Try crushing a vitamin B-12 tablet and dissolving it in vinegar. The solution will glow bright yellow under under a black light. - Antifreeze
Manufacturers purposedly include fluorescent additives in antifreeze fluid so that black lights can be used to find antifreeze splashes to help invesitagors reconstruct automobile accident scenes. - Laundry Detergents
Some of the whiteners in detergent work by making your clothing a bit fluorescent. Even though clothing is rinsed after washing, residues on white clothing cause it to glow bluish-white under a black light. Blueing agents and softening agents often contain fluorescent dyes, too. The presence of these molecules sometimes causes white clothing to appear blue in photographs.
"Ultraviolet light" generally refers to electromagnetic radiation with wavelengths in the range of 10 to 400 nanometers. This is subdivided into:
- UV-A = 315 to 400 nM.
345 to 400 nM = used for "Black light" effects.
315 to 345 nM = are used for suntanning (some sun lamps also generate UV-B) - UV-B = 280 to 315 nanometers. Hazardous! Largely responsible for sunburn.
- UV-C = 200 to 280 nM. Dangerous! Used to kill germs.
- Vacuum Ultraviolet - 10 to 200 nM.
- The peak absorption wavelengths are 325, 365 and 405 nanometers. Our spectronics light is 365 nanometers and the vector 7 flashlight is 405 nanometers. The spectronic light is availabe in two bulbs: concentrated spot and broadbeam spot. The Vector 7 is a concentrated spot while the U-View light is a broadbeam spot.
-Scott Warrington
